- Details
- Written by: Germán Fernández
- Category: Chemical Reactors
- Hits: 1366

Chemical reactors are devices or equipment used in the chemical industry and laboratories to carry out controlled chemical reactions. These devices are specifically designed to allow reactants to mix, react, and produce desired chemicals efficiently and in a controlled manner. There are various types of chemical reactors, and the choice of the appropriate type depends on the nature of the reaction and the goals of the process. Below are descriptions of some common types of chemical reactors:
1. Continuous Stirred Tank Reactors (CSTR): In these reactors, reactants are continuously introduced into a tank and stirred constantly. The reaction takes place in the tank, and the products are continuously removed. CSTRs are used in reactions that require a constant residence time and in steady-state processes.
2. Fixed-Bed Reactors: In these reactors, reactants flow through a solid catalytic bed or solid reactive material. These reactors are used in heterogeneous catalysis processes, such as hydrocarbon cracking in the petroleum industry.
3. Fluidized-Bed Reactors: In these reactors, a stream of gas is used to maintain a bed of solid particles in suspension. They are used in processes involving heterogeneous gas-phase reactions, such as ammonia synthesis.
- Details
- Written by: Germán Fernández
- Category: Chemical Reactors
- Hits: 1198
The reaction rate $r_i$ is defined as the number of moles consumed or generated per unit of time and reaction mixture volume. \begin{equation} r_i=\frac{1}{V}\frac{dN_i}{dt} \end{equation}
In many chemical reactions, the reaction rate can be expressed as the product of a temperature-dependent factor (kinetic constant) and another factor dependent on the concentrations of reactants.
Consider the reaction $aA+bB\rightarrow cC +dD$, the rate is given by $(-r_A)=-\frac{dc_A}{dt}=kc_A^{\alpha}c_B^{\beta}$, where $\alpha$ and $\beta$ are the partial orders with respect to reactants A and B. The sum of both partial orders gives the overall or global order of the reaction.
- Details
- Written by: Germán Fernández
- Category: Chemical Reactors
- Hits: 2187
The conversion \(x_A\) is defined as the fraction of reactant A that is transformed into a product. We can obtain an expression for the conversion from the equation that gives the moles of unreacted reactant A:
\(N_A = N_{A0}(1-x_A) \rightarrow x_A = \frac{N_{A0}-N_A}{N_{A0}}\).
- Details
- Written by: Germán Fernández
- Category: Chemical Reactors
- Hits: 1222
In gas-phase reactions, volume variations can occur as the reaction progresses. In this situation, we will use a simplified expression that gives the volume as a function of conversion: \(V = V_0(1 + \epsilon_Ax_A)\), where \(\epsilon_A\) is the relative volume change factor with the conversion of reactant A.
\(\epsilon_A = \frac{V(x_A=1) - V(x_A=0)}{V(x_A=0)}\)
- Details
- Written by: Germán Fernández
- Category: Chemical Reactors
- Hits: 1644
The Arrhenius law gives the dependence of the equilibrium constant on temperature. \begin{equation} k=Ae^{-E_a/RT} \end{equation} A is the frequency factor, related to the frequency with which the activated complex decomposes into products.
Where:
- is the reaction rate constant,
- is the pre-exponential factor or frequency factor,
- is the activation energy of the reaction,
- is the ideal gas constant, and
- is the temperature in kelvins.
- Details
- Written by: Germán Fernández
- Category: Chemical Reactors
- Hits: 1730
A discontinuous ideal reactor, also known as a batch reactor, is a type of chemical reactor in which reactants are loaded into the reactor, the reaction is allowed to occur for a specific period, and then the resulting product is discharged. This type of reactor operates in a batch cycle, meaning there is an initial loading of reactants, followed by a reaction period, and finally, the discharge of the product.
Key features of a discontinuous ideal reactor:
-
Batch Loading: Reactants are introduced into the reactor in a finite quantity at the beginning of each operating cycle.
-
Batch Operation: The chemical reaction occurs for a finite and predefined period.
-
Product Discharge: After completing the reaction, the product is removed from the reactor.
-
No Input or Output During Reaction: During the reaction period, no new reactants are introduced, and no products are withdrawn.
-
Condition Control: Conditions such as temperature and pressure can be controlled and adjusted during the reaction process.
-
Flexibility: This type of reactor is suitable for processes that require specific conditions during the reaction and can benefit from batch production.
It is important to note that, although the discontinuous ideal reactor provides some flexibility and control in production, it is not suitable for processes requiring continuous and constant production. Additionally, the total operation time includes both reaction time and loading/unloading time, which can impact the efficiency of the process compared to continuous flow reactors.
The reactants are introduced into the reactor, mixed, allowed to react for a certain time, and finally, the resulting mixture is discharged. The composition varies over time but is uniform throughout the reactor.
Material balance on the reactor:
Input = Output + Disappearance + Accumulation
- Input = Output = 0
- Disappearance of A by reaction: $(-r_A)V$
- Accumulation of A: $N_A=N_{A0}(1-x_A)$, differentiating with respect to t, $\frac{dN_A}{dt}=-N_{A0}\frac{dx_A}{dt}$
Substituting into the material balance:
\begin{equation} 0=(-r_A)V-N_{A0}\frac{dx_A}{dt} \end{equation}
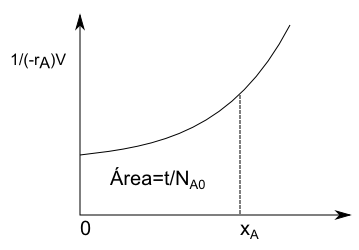
Separating variables:
\begin{equation} dt=N_{A0}\frac{dx_A}{(-r_A)V}\;\rightarrow\;\int_{0}^{t}dt=N_{A0}\int_{0}^{x_A}\frac{dx_A}{(-r_A)V} \end{equation}
The design equation for a BR is then:
\begin{equation} t=N_{A0}\int_{0}^{x_A}\frac{dx_A}{(-r_A)V} \end{equation}
Read more: Design equation for an ideal discontinuous reactor
- Details
- Written by: Germán Fernández
- Category: Chemical Reactors
- Hits: 3179

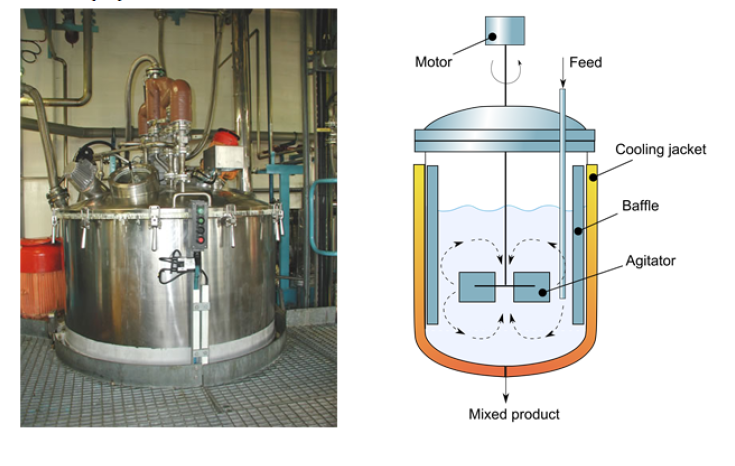
A Continuous Stirred Tank Reactor (CSTR) is a type of chemical reactor designed to conduct continuous chemical reactions in liquid or gaseous phase. Below, its main characteristics are described:
1. Continuous Mixing: The CSTR maintains a continuous and uniform mixture of reactants inside. This is achieved through the use of an agitation system, such as a propeller or a paddle, ensuring constant dispersion of the reactants.
2. Continuous Inflow and Outflow: Unlike a batch reactor, in a CSTR, reactants continuously enter and exit throughout the process. This allows uninterrupted operation and constant product production.
3. Homogeneous Conditions: The constant mixing ensures that conditions inside the reactor, such as temperature and concentration, are practically uniform throughout the reactor's volume.
4. Variable Control: Various variables, including temperature, pressure, and input/output flow rates, can be controlled and adjusted to optimize reaction conditions.
5. Versatile Applications: CSTRs are versatile and find use in a wide range of industrial processes, including chemical synthesis, chemical product manufacturing, fermentation in the food industry, and more.
6. Residence Time: The time a reactant spends inside the reactor, known as residence time, is crucial in determining reaction efficiency. This residence time can be adjusted by controlling the inflow and outflow.
7. Mixing Efficiency: Mixing efficiency is a crucial feature of CSTR, as rapid and homogeneous mixing enhances reaction efficiency and prevents the formation of concentration gradients.
8. Mathematical Modeling: CSTRs are often described through differential equations that account for reaction kinetics and operating conditions. This aids in understanding and predicting reactor behavior.
In summary, a CSTR is a fundamental tool in chemical engineering for conducting continuous and controlled chemical reactions, providing flexibility and efficiency in a variety of industrial applications.
The content of the reactor is perfectly stirred, and its composition is the same at every instant at all points in the reactor. The outlet stream of this reactor has the same composition as the fluid contained in it.
- $c_{A0}$: Initial concentration of reactant A ($mol/m^3$).
- $x_{A0}$: Initial conversion.
- $Q_v$: Volumetric flow rate ($m^3/s$).
- $F_{A0}$: Molar flow of reactant A at the inlet (mol/s).
- $F_{A0}=c_{A0}Q_v$
- Reaction $A\rightarrow P$ with rate $(-r_A)$.
Material balance to the reactor: Accumulation = Inlet - Outlet - Disappearance
- Accumulation of A = 0 (No accumulation, everything that enters exits).
- Inlet of A = $F_{A0}$
- Outlet of $A = F_{A}=F_{A0}(1-x_A)$
- Disappearance of A by reaction = $(-r_A)V$
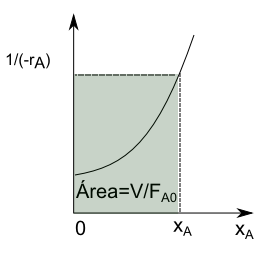
Substituting into the material balance:
\begin{equation} 0=F_{A0}-F_{A0}(1-x_A)-(-r_A)V \end{equation}
Simplifying this last expression, we obtain the design equation of the CSTR
\begin{equation} \frac{V}{F_{A0}}=\frac{x_A}{(-r_A)} \end{equation}
Read more: Design equation of an ideal continuous complete mixing reactor (CSTR)
- Details
- Written by: Germán Fernández
- Category: Chemical Reactors
- Hits: 3887
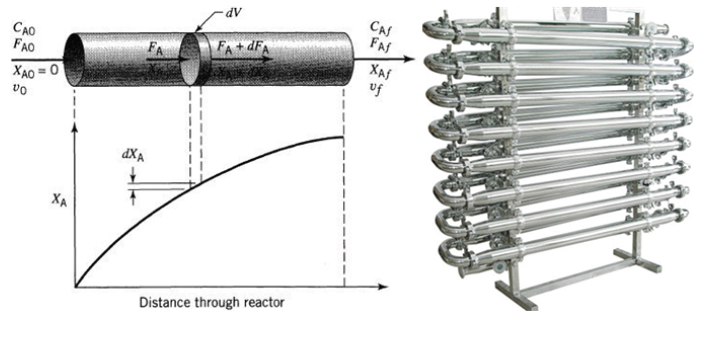
A Piston Flow Reactor (PFR) or Tubular Flow Reactor is a type of chemical reactor in which reactants flow continuously through a tube or a system of pipes, and chemical reactions occur as the reactants move through the reactor. This type of reactor is characterized by having a constant and stationary flow of reactants through the system.
Here are some key features of a PFR:
-
Continuous Flow: Unlike other types of reactors, such as batch reactors, in a PFR, reactants continuously enter the system, and products are continuously extracted.
-
Residence Time Distribution: The time a reactant spends inside the reactor, known as residence time, varies along the reactor. Each reactant particle has a different residence time, allowing for a distribution of residence time.
-
Concentration Profile: Due to the constant flow, the concentration profile along the reactor changes as reactants react and convert into products. This contrasts with an ideal piston flow reactor, where the concentration of reactants remains constant in any cross-sectional area of the reactor.
-
Steady-State Reactions: Under ideal conditions, the PFR operates in a steady state, meaning that the system properties do not change over time. However, practical situations may lead to variations in reactor conditions.
-
Applications: PFRs are used in various industrial applications, such as chemical synthesis, pharmaceutical product manufacturing, polymer synthesis, and other chemical reactions on an industrial scale.
It is important to note that while the ideal piston flow reactor model is often used for theoretical and design purposes, in practice, PFRs may deviate from this ideal model due to factors such as imperfect mixing and variability in operating conditions.
Read more: Design equation of an ideal plug flow reactor (PFR)


