
A Continuous Stirred Tank Reactor (CSTR) is a type of chemical reactor designed to conduct continuous chemical reactions in liquid or gaseous phase. Below, its main characteristics are described:
1. Continuous Mixing: The CSTR maintains a continuous and uniform mixture of reactants inside. This is achieved through the use of an agitation system, such as a propeller or a paddle, ensuring constant dispersion of the reactants.
2. Continuous Inflow and Outflow: Unlike a batch reactor, in a CSTR, reactants continuously enter and exit throughout the process. This allows uninterrupted operation and constant product production.
3. Homogeneous Conditions: The constant mixing ensures that conditions inside the reactor, such as temperature and concentration, are practically uniform throughout the reactor's volume.
4. Variable Control: Various variables, including temperature, pressure, and input/output flow rates, can be controlled and adjusted to optimize reaction conditions.
5. Versatile Applications: CSTRs are versatile and find use in a wide range of industrial processes, including chemical synthesis, chemical product manufacturing, fermentation in the food industry, and more.
6. Residence Time: The time a reactant spends inside the reactor, known as residence time, is crucial in determining reaction efficiency. This residence time can be adjusted by controlling the inflow and outflow.
7. Mixing Efficiency: Mixing efficiency is a crucial feature of CSTR, as rapid and homogeneous mixing enhances reaction efficiency and prevents the formation of concentration gradients.
8. Mathematical Modeling: CSTRs are often described through differential equations that account for reaction kinetics and operating conditions. This aids in understanding and predicting reactor behavior.
In summary, a CSTR is a fundamental tool in chemical engineering for conducting continuous and controlled chemical reactions, providing flexibility and efficiency in a variety of industrial applications.
The content of the reactor is perfectly stirred, and its composition is the same at every instant at all points in the reactor. The outlet stream of this reactor has the same composition as the fluid contained in it.
- $c_{A0}$: Initial concentration of reactant A ($mol/m^3$).
- $x_{A0}$: Initial conversion.
- $Q_v$: Volumetric flow rate ($m^3/s$).
- $F_{A0}$: Molar flow of reactant A at the inlet (mol/s).
- $F_{A0}=c_{A0}Q_v$
- Reaction $A\rightarrow P$ with rate $(-r_A)$.
Material balance to the reactor: Accumulation = Inlet - Outlet - Disappearance
- Accumulation of A = 0 (No accumulation, everything that enters exits).
- Inlet of A = $F_{A0}$
- Outlet of $A = F_{A}=F_{A0}(1-x_A)$
- Disappearance of A by reaction = $(-r_A)V$
Substituting into the material balance:
\begin{equation} 0=F_{A0}-F_{A0}(1-x_A)-(-r_A)V \end{equation}
Simplifying this last expression, we obtain the design equation of the CSTR
\begin{equation} \frac{V}{F_{A0}}=\frac{x_A}{(-r_A)} \end{equation}
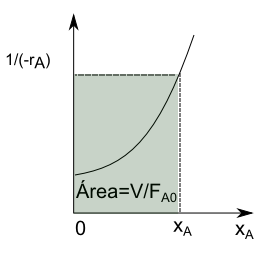
In systems of constant density, $c_A=c_{A0}(1-x_a)$, solving for $x_A$, $x_A=\frac{c_{A0}-c_A}{c_{A0}}$.
The design equation becomes,
\begin{equation} \frac{Vc_{A0}}{F_{A0}}=\frac{c_{A0}-c_A}{(-r_A)} \end{equation}
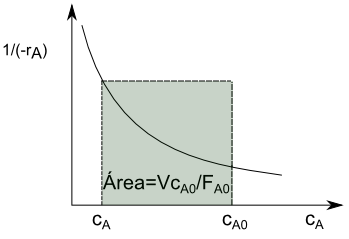
Spatial time and space velocity
The spatial time $\tau$ is defined as the time required to process a feed volume equal to the reactor volume.
\begin{equation} \tau=\frac{V}{Q_v}=\frac{Vc_{A0}}{F_{A0}} \end{equation}
The space velocity (S) is defined as the number of feed volumes that can be treated in a unit of time, measured in reactor volumes. Space velocity is the inverse of spatial time.
\begin{equation} S=\frac{1}{\tau} \end{equation}
A Continuous Stirred Tank Reactor (CSTR) is used in a variety of chemical reactions where continuous and controlled operation is desired. Some examples of reactions to which a CSTR can be applied include:
- Chemical Synthesis Reactions:
- Production of basic chemical products.
- Synthesis of organic compounds.
- Manufacturing of polymers.
- Fermentation Reactions:
- Production of food products, such as dairy fermentation.
- Fermentation for the production of chemical products, such as lactic acid.
- Bioremediation Reactions:
- Decomposition of environmental pollutants by microorganisms.
- Neutralization Reactions:
- Neutralization of acids and bases for the production of salts.
- Hydrolysis Reactions:
- Hydrolysis of esters for the production of alcohols and acids.
- Esterification Reactions:
- Synthesis of esters from acids and alcohols.
-
Polymerization Reactions:
- Polymerization of monomers for the production of polymers.
-
Thermal Decomposition Reactions:
- Decomposition of compounds through heat.
-
Ion Exchange Reactions:
- Exchange of ions in aqueous solutions.
-
Oxidation-Reduction Reactions:
- Oxidation or reduction of chemical compounds.
-
Hydrogenation Reactions:
- Hydrogenation of unsaturated compounds.
These are just examples, and the applicability of the CSTR may vary depending on the specific conditions of each reaction and process requirements. The efficient design and operation of the CSTR depend on factors such as reaction kinetics, properties of reactants and products, and desired operating conditions.


