
A chemical process is a set of chemical and/or physical operations aimed at transforming initial materials into different final products.
It is a sequence of steps or operations carried out with the purpose of transforming one or more chemical substances into one or several different substances. These processes may involve a variety of activities, such as mixing, heating, cooling, separating, reacting, purifying, and manipulating chemical substances to obtain desired products. Chemical processes are fundamental in the chemical, pharmaceutical, petrochemical, food, and various other industries, and are used for the production of a wide range of chemicals, materials, and end products. These processes are often carried out following specific protocols and procedures with the aim of safely and efficiently obtaining chemicals with desired properties.
The production of caustic soda flakes, also known as sodium hydroxide flakes, is a chemical process that involves the conversion of common chemicals, such as salt and lime, into solid sodium hydroxide in the form of flakes. This process can be carried out following the following general steps:
1. Raw material acquisition:
Sodium chloride (common salt): Sodium chloride is the main raw material for the production of caustic soda flakes. It can be obtained from natural sources such as salt flats or salt mines or through processes of seawater evaporation.
2. Electrolysis of brine:
Brine is prepared by dissolving sodium chloride in water. The brine undergoes an electrolysis process in an electrolytic cell. In this process, an electric current is applied through the brine, leading to the breakdown of sodium chloride (NaCl) into its basic components: sodium ions (Na+) and chloride ions (Cl-).
Sodium ions migrate to the cathode, where they are reduced and combine with water to form sodium hydroxide (NaOH), while chloride ions move to the anode.
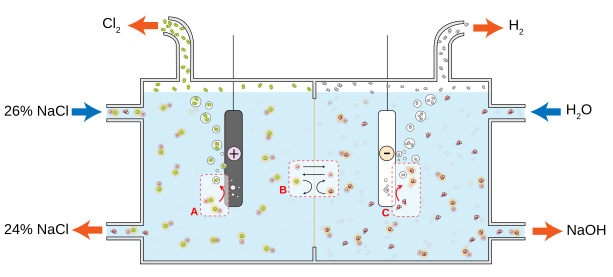
3. Collection and purification of sodium hydroxide:
The sodium hydroxide produced at the cathode is collected and purified to remove impurities and concentrate the solution.
4. Crystallization:
The concentrated solution of sodium hydroxide is cooled, causing the crystallization of sodium hydroxide in the form of flakes.
5. Drying:
Sodium hydroxide flakes are dried to remove residual moisture and obtain the final product in flake form.
It is important to note that this is a simplified process, and the production of caustic soda flakes may involve specific equipment and controls to ensure product quality and safety. Additionally, it is a process that requires in-depth knowledge of chemistry and chemical engineering to be carried out efficiently and safely in an industrial plant.
The production of caustic soda flakes, as mentioned earlier, involves the conversion of sodium chloride (common salt, NaCl) into sodium hydroxide (NaOH) in the form of flakes. The main chemical product in this process is sodium hydroxide, which is a solid substance in the form of flakes and is a widely used strong base in the chemical industry. Sodium hydroxide has various applications, such as in the manufacturing of cleaning products, pharmaceuticals, food products, and in paper production, among others.
In addition to sodium hydroxide, byproducts or waste products, such as chlorine gas (Cl2), can be generated in the production of caustic soda flakes at the anode during brine electrolysis. Chlorine is also an important chemical product and is used in numerous applications, such as the production of chlorinated chemicals, disinfectants, and plastics.
It is important to highlight that the chemical process must be designed and controlled to minimize the generation of unwanted byproducts and waste, and to comply with applicable environmental and safety regulations. Therefore, the production of caustic soda flakes focuses on obtaining sodium hydroxide as the main product and, as far as possible, on the responsible capture and management of byproducts.


